About The Clinical Eye Floater Studies
Get a closer look at two groundbreaking clinical studies and their impressive results, demonstrating the effectiveness of key ingredients in addressing eye floaters.
The studies cited below can be found online under the aegis of the NIH Natural Library of Medicine. Links to the studies are provided below. Study results are an indication of possibility. They do not imply that everyone who takes similar supplements will obtain the same results. Float-Away is not intended to diagnose, treat, cure, or prevent any disease.
STUDY 1
Mixed Fruit Enzyme (MFE) Supplement for Vitreous Floaters
When did the study take place?
Conducted between September and December 2017; published in November 2022.
Where did the study take place?
Conducted at Kaohsiung Armed Forces General Hospital in Taiwan.
What company or lab conducted the study?
Led by researchers from Tajen University, Meiho University, and the National Defense Medical College in collaboration with Kaohsiung Armed Forces General Hospital.
What was the formula used?
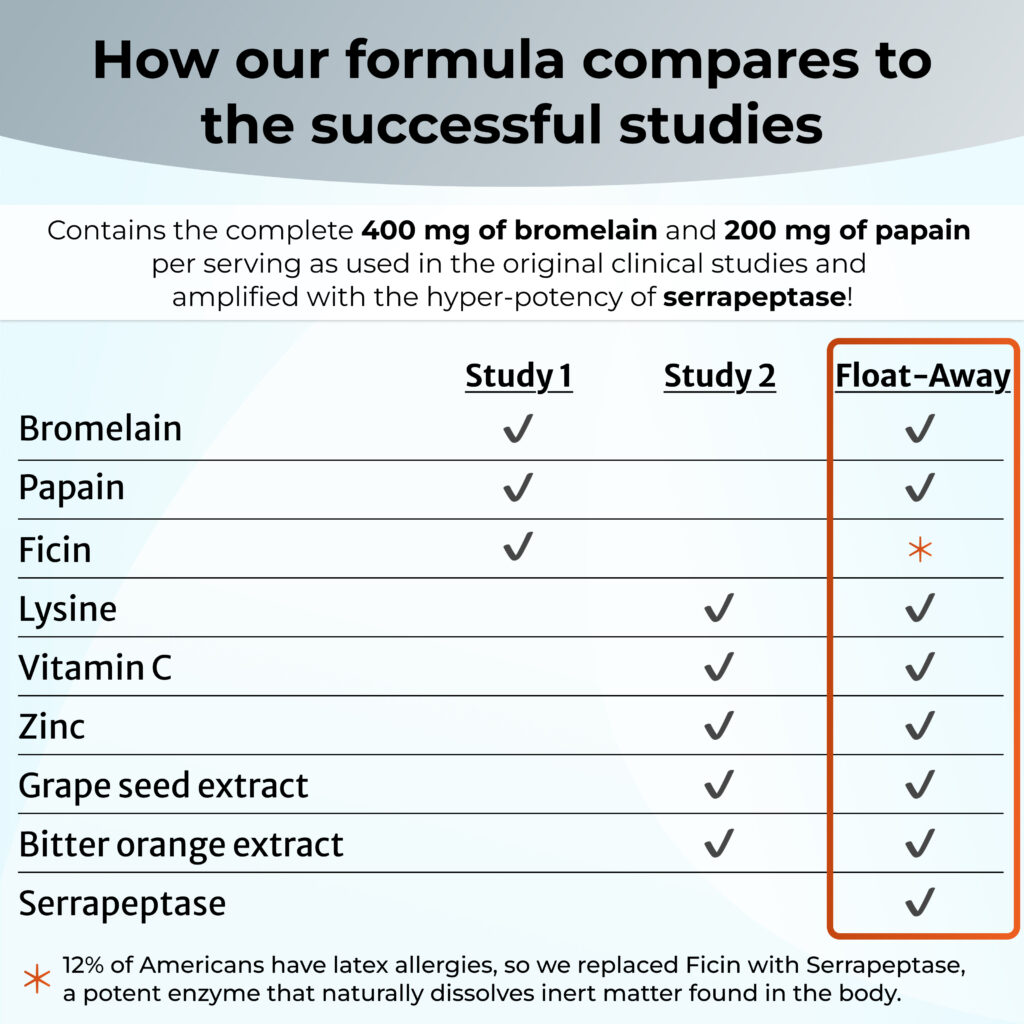
Mixed Fruit Enzyme (MFE) formula containing:
– 190 mg bromelain (pineapple enzyme)
– 95 mg papain (papaya enzyme)
– 95 mg ficin (fig enzyme)
How many people participated?
224 patients:
– 160 participants with primary floaters (spontaneous vitreous opacities, SVO).
– 64 participants with VH (*Vitreous hemorrhage)-induced floaters.
*Vitreous hemorrhage – a sometimes serious seepage of blood into the vitreous often resulting in pronounced floaters and other vision impairments
What was the success rate?
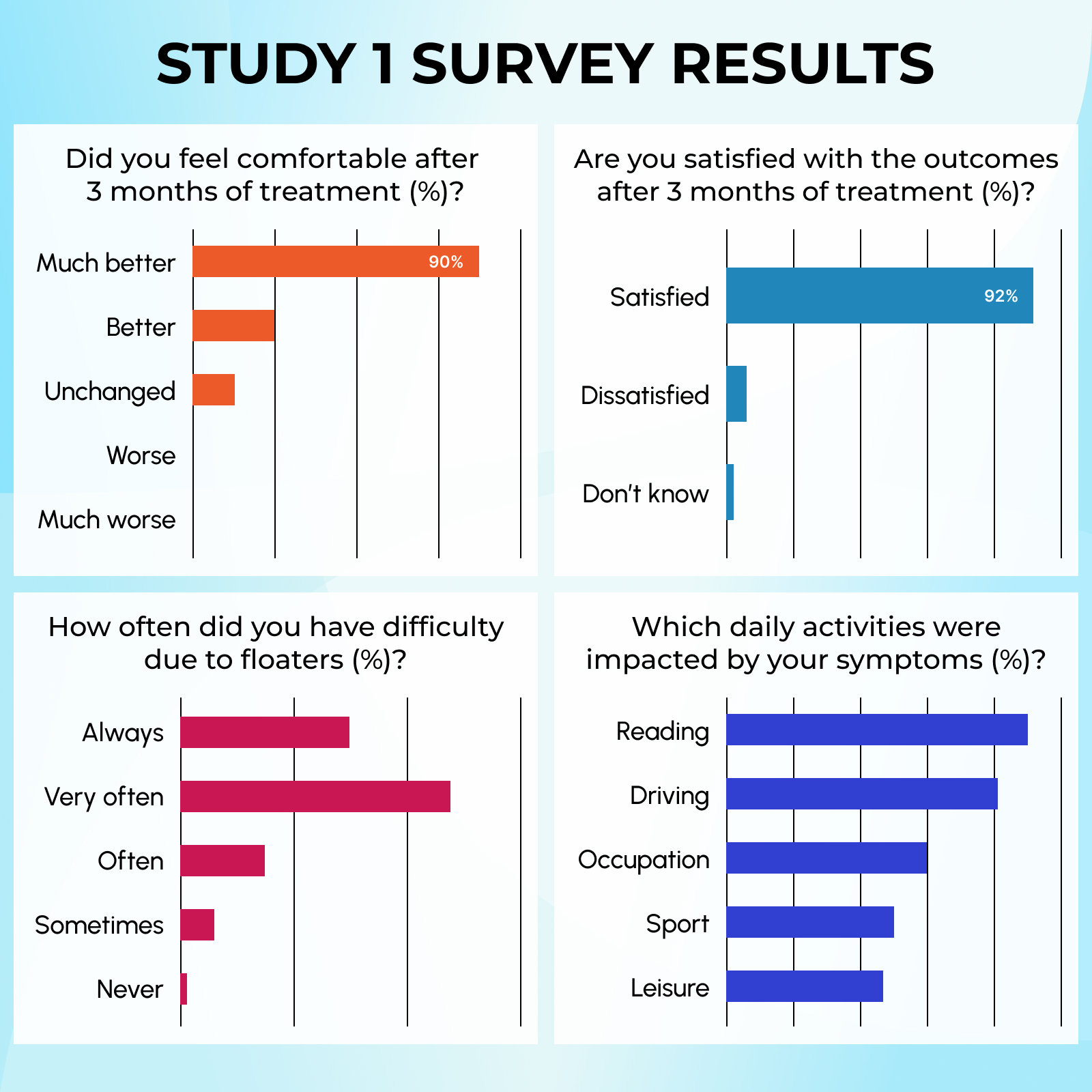
Within 3 months:
Primary floaters (SVO):
– 55% disappearance with 1 serving/day.
– 62.5% disappearance with 2 servings/day.
– 70% disappearance with 3 servings/day.
VH-induced floaters:
– 18% disappearance with 1 servings/day.
– 25% disappearance with 2 servings/day.
– 56% disappearance with 3 servings/day.
Significant improvement in corrected distance visual acuity for VH-induced floaters correlated with the number of servings per day.
Participants saw results within 3 months

2 SERVINGS DAILY:
62.5% began to see significant symptom reduction
3 SERVINGS DAILY:
70% began to see significant symptom reduction
STUDY 2
Targeted Micronutrient Formulation for Vitreous Floaters
When did the study take place?
Conducted in 2021; published in October 2021.
Where did the study take place?
Conducted at the Nutrition Research Centre Ireland, Waterford Institute of Technology, Ireland.
What company or lab conducted the study?
The study was led by researchers from the Nutrition Research Centre Ireland and supported by the Institute of Eye Surgery, Waterford, Ireland.
What was the formula used?
The active supplement contained:
– 125 mg L-lysine
– 40 mg vitamin C
– 26.3 mg Vitis vinifera (grape seed) extract
– 5 mg zinc
– 100 mg Citrus aurantium (bitter orange) extract
How many people participated?
61 patients were enrolled, randomized into two groups:
31 in the active group and 30 in the placebo group.
What was the success rate?
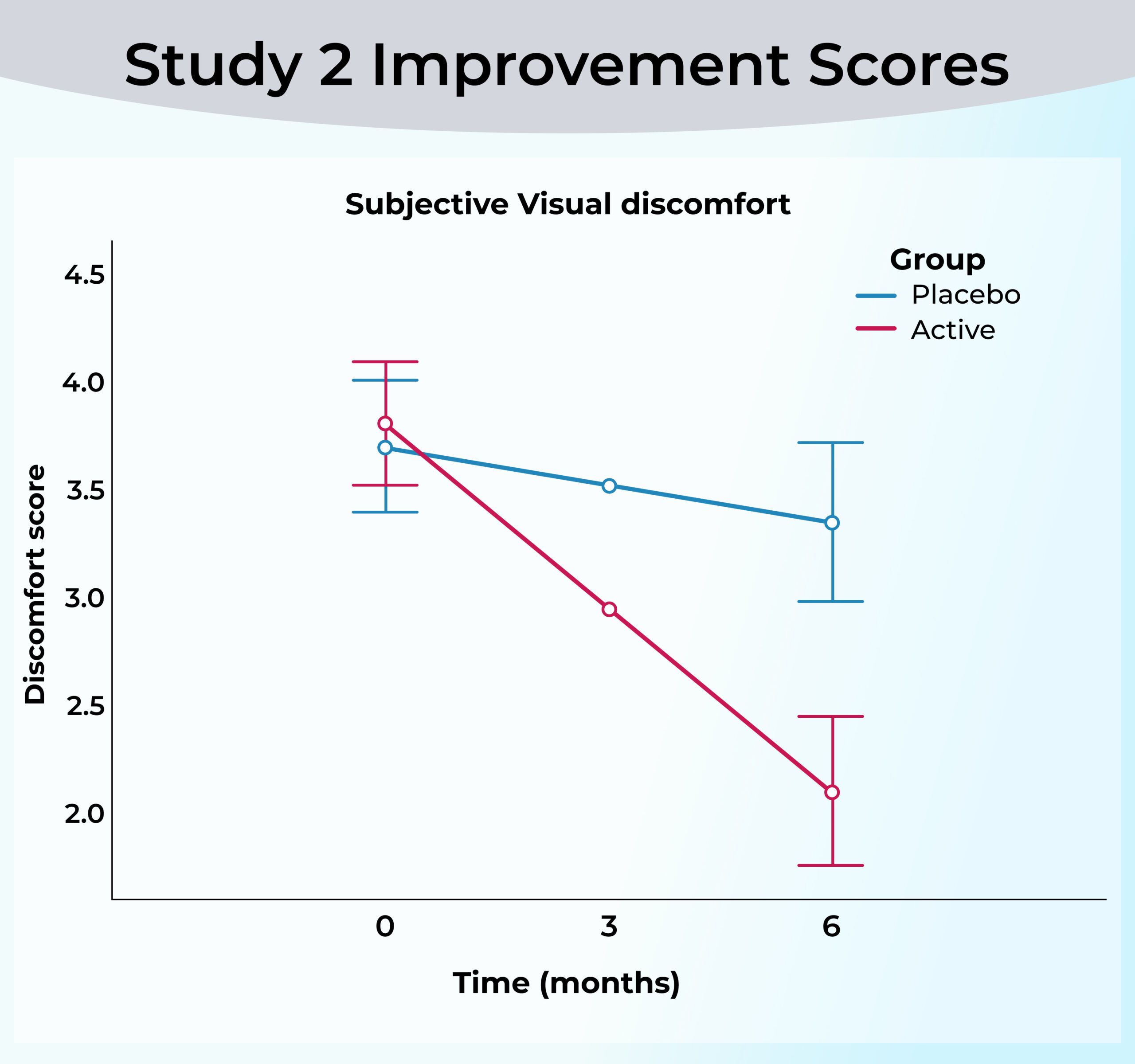
Within 6 months:
Visual discomfort improvement: 66.6% of participants in the active group reported a decrease in floater-related discomfort.
Vitreous opacity obstructed vision: Significant reduction in the active group, with the placebo group showing no significant improvement.
Contrast sensitivity: Significant improvement in photopic functional contrast sensitivity in the active group.
Approximately 2/3 of participants began to see satisfying changes in less than 3 months!